Case Study
USHA SHARMA – TVN SPECIALIST NURSE, TEAM LEADER, The royal Wolverhampton NHS Trust.
Introduction:
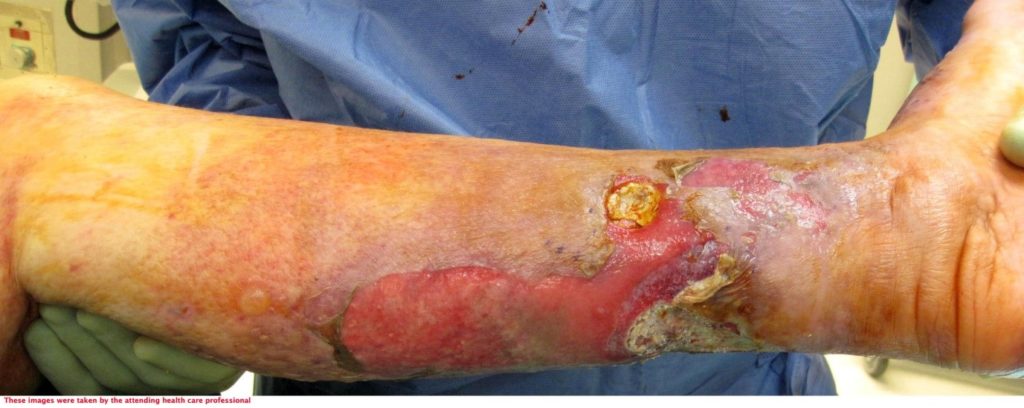
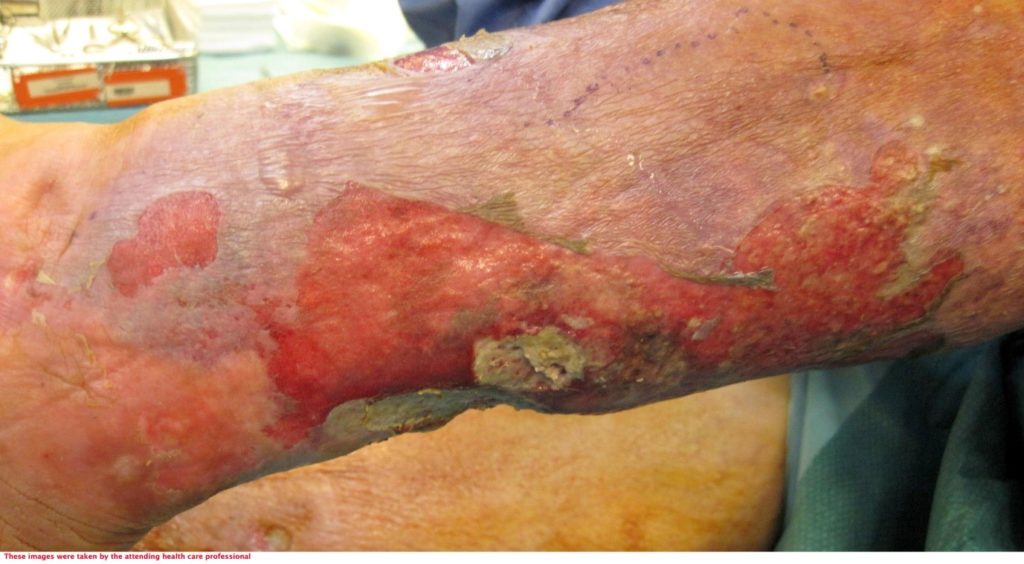
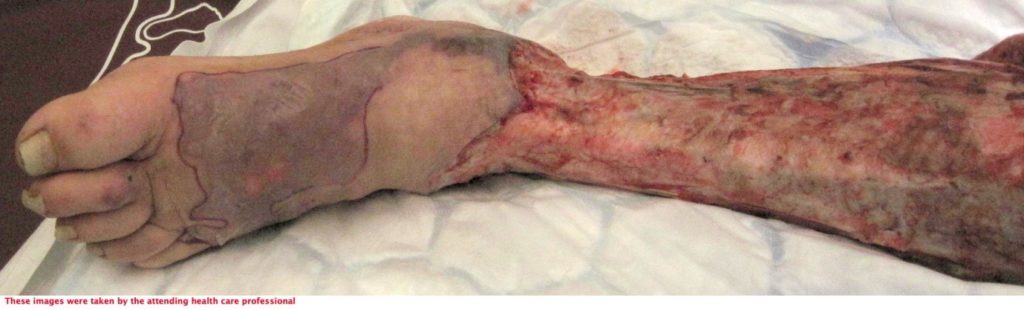
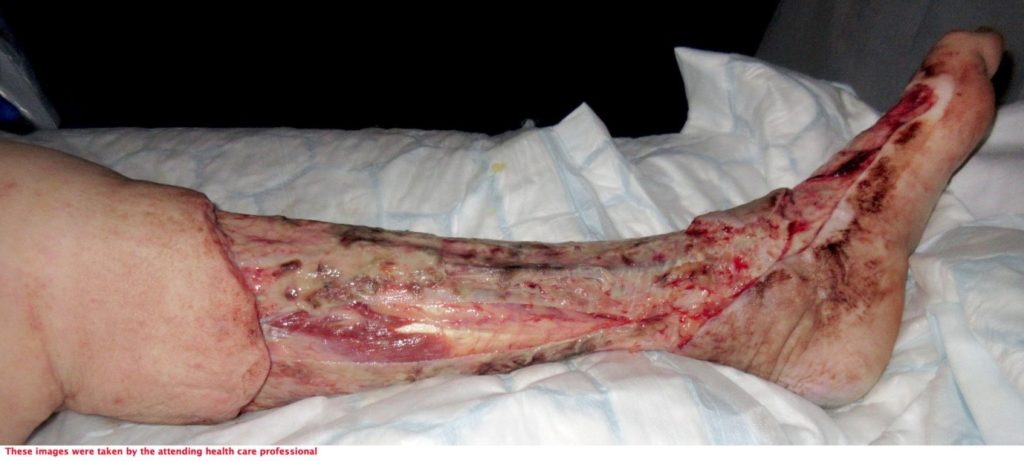
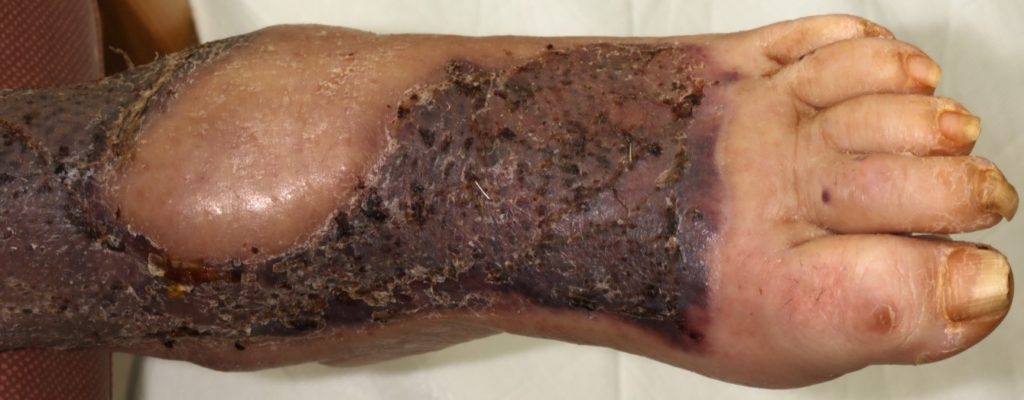
72-year-old lady admitted via ED with sepsis. First impression in medical notes was of cellulitis of legs (figure 1& 2). The lady had a past medical history of IBS, Asthma, CKD, Pyoderma gangrenosum, Osteoarthritis, vasculitis on renal biopsy, Hyperthyroidism, Hypertension and was Diabetic.
Method:
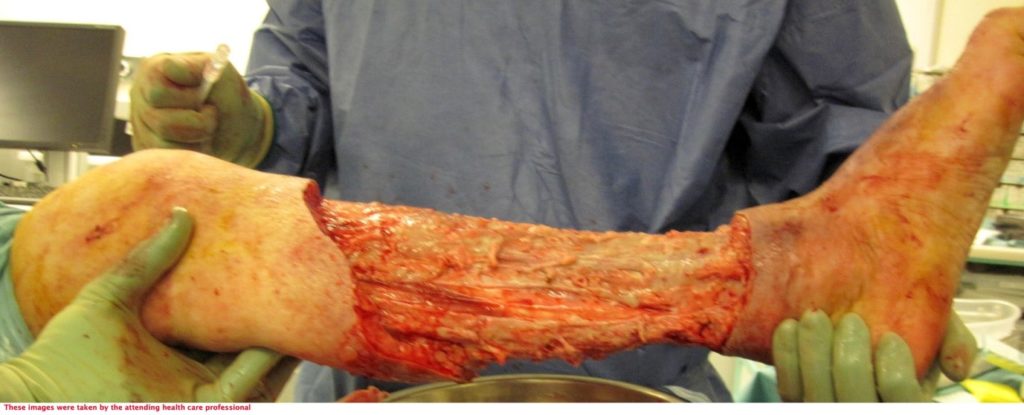
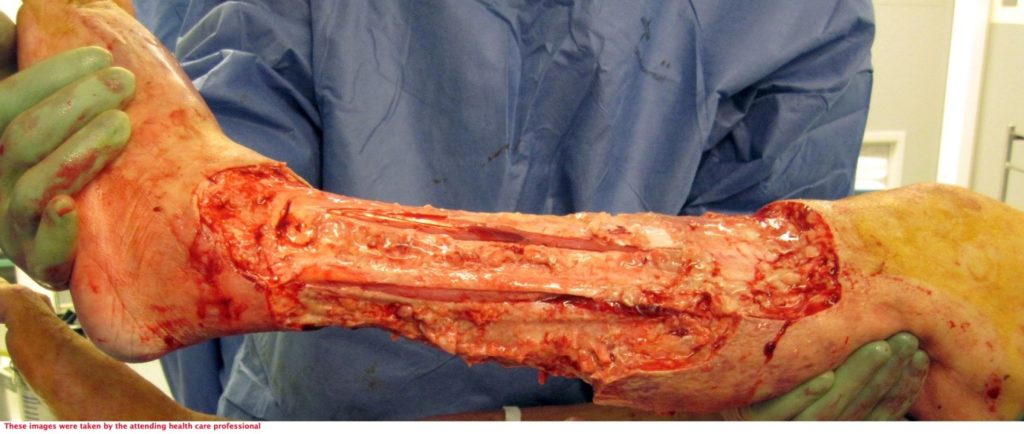
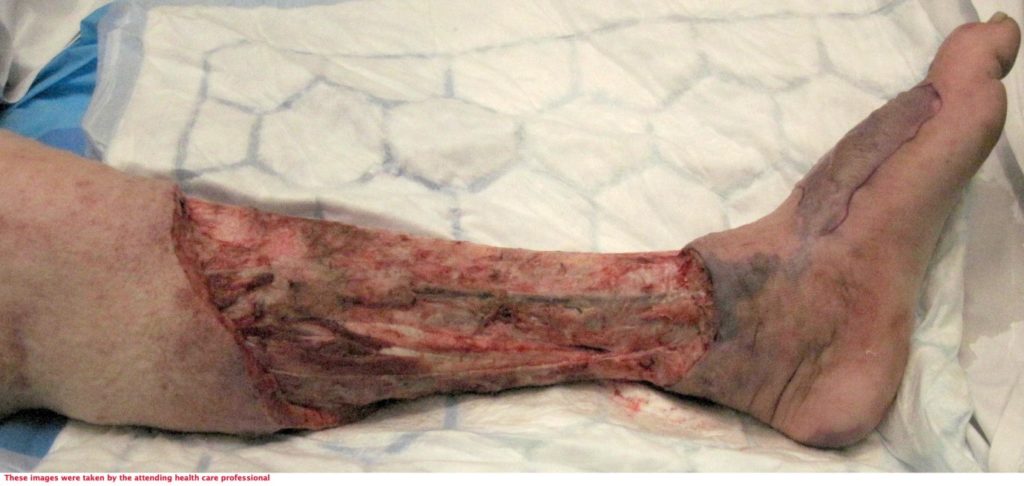
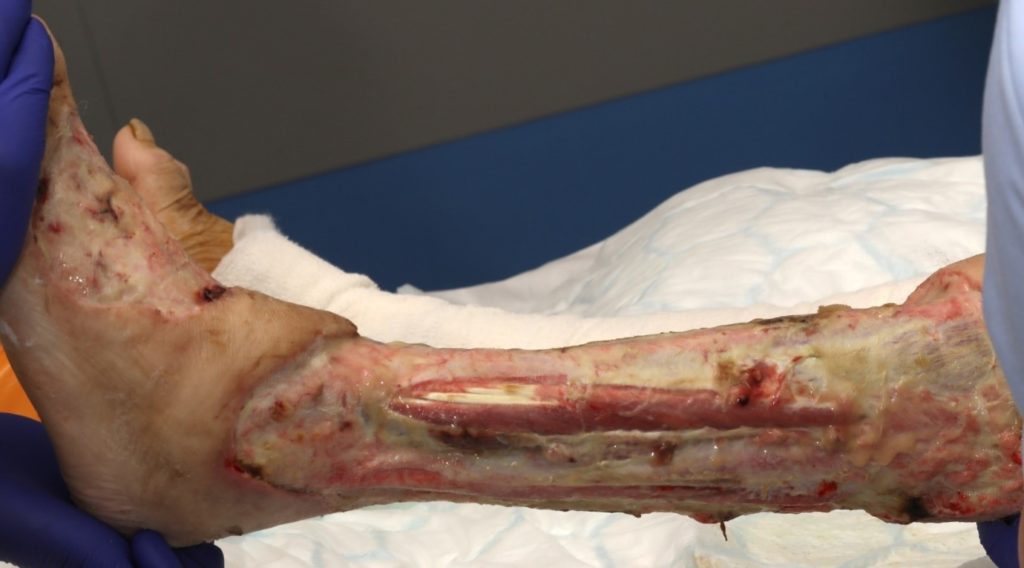
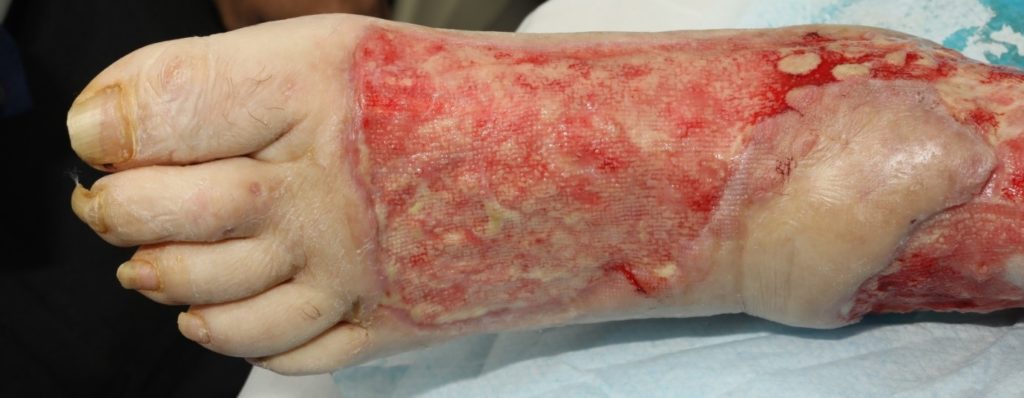
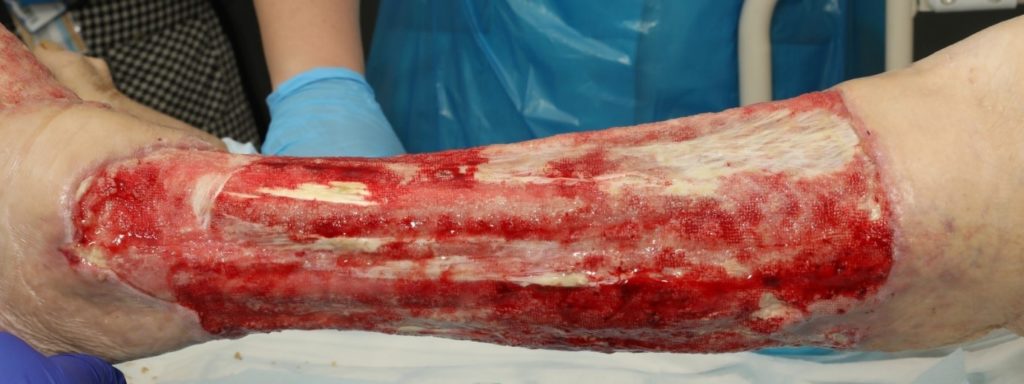
The patient underwent two debridement’s over the course of two days (Figure 3 & 4) completed in theatre. A second debridement was required due to extensive spread of Necrotising Fasciitis, extending to the foot (Figure 5 & 6).
Referral received from ICCU, to TVN team to review leg wound following extensive surgical debridement
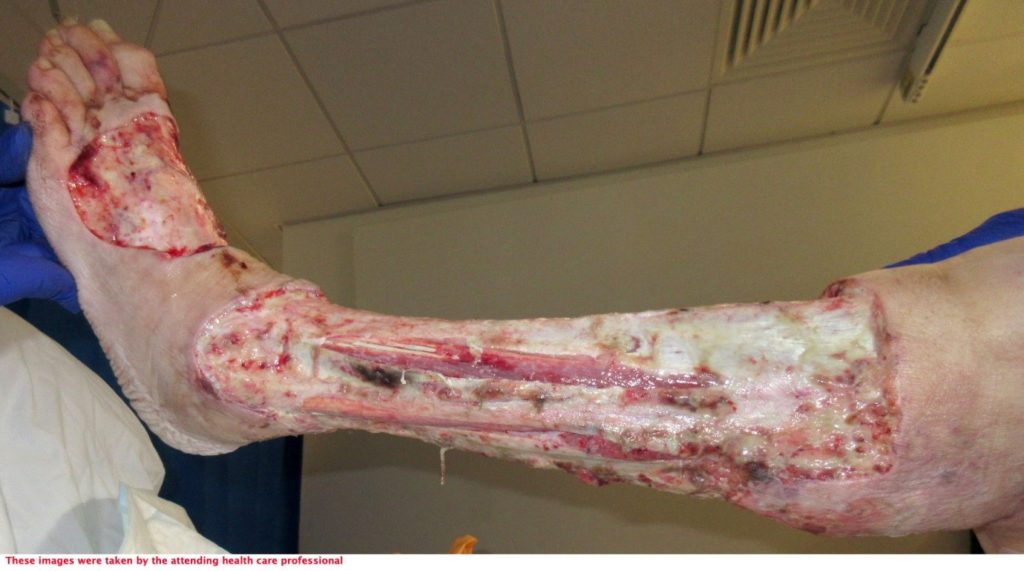
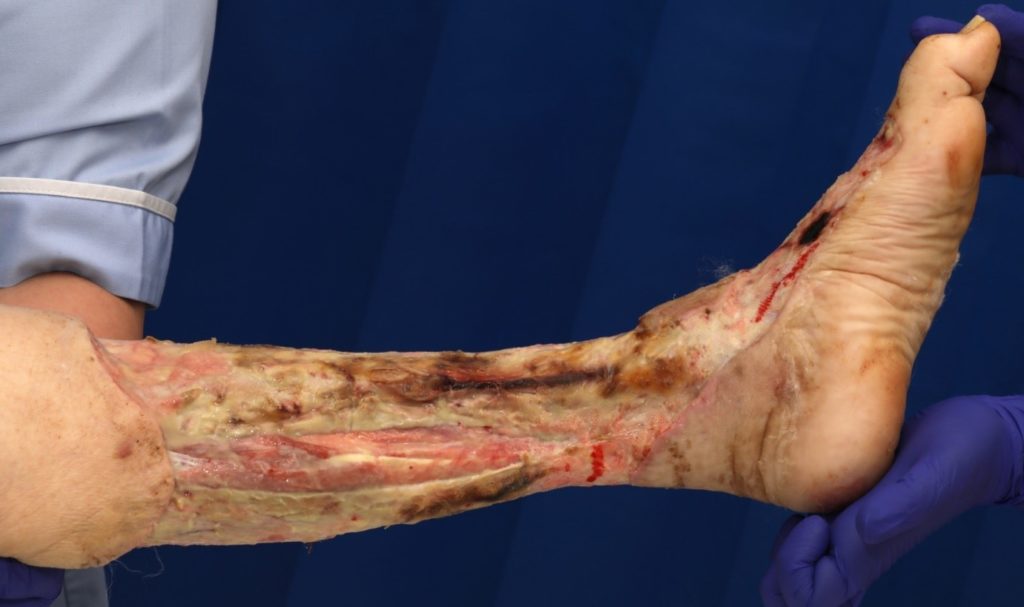
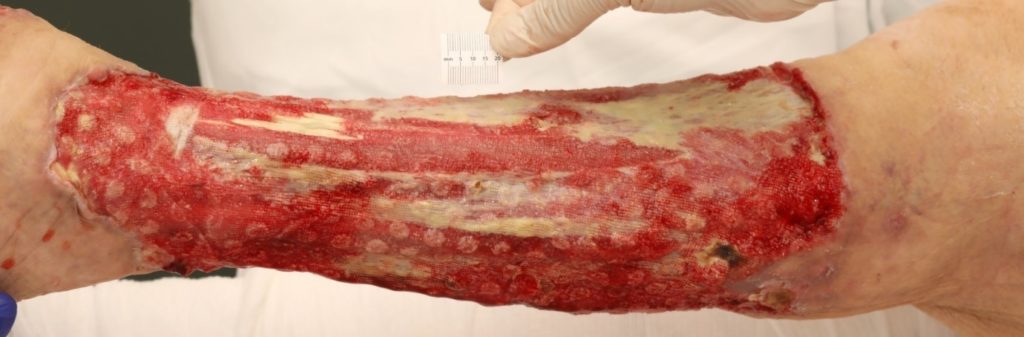
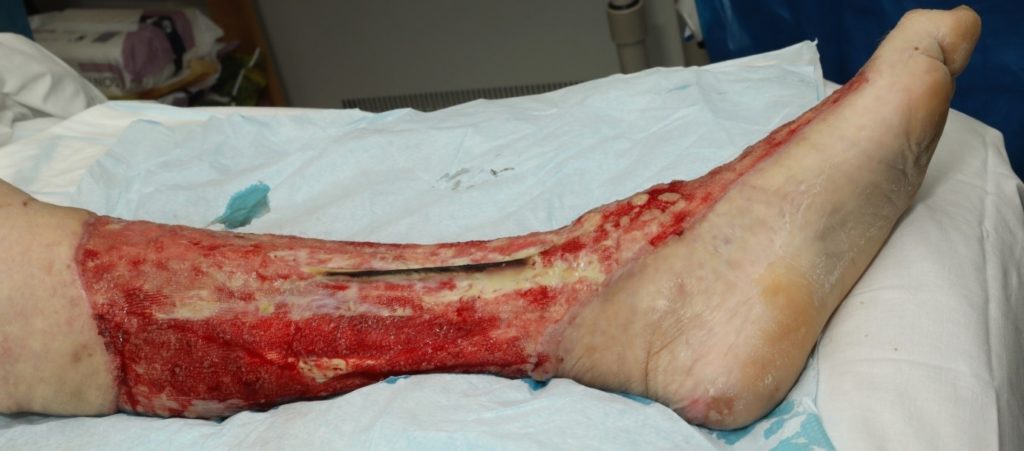
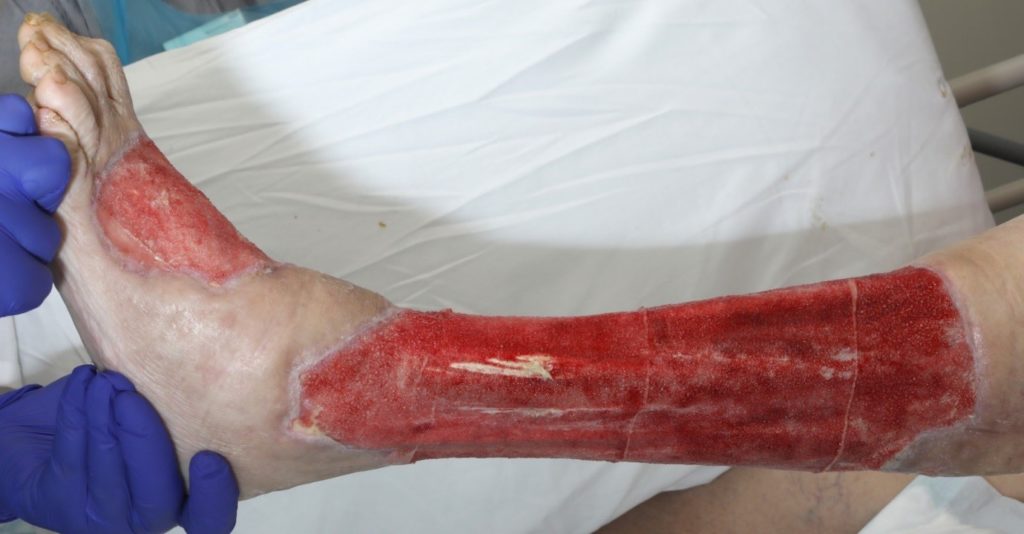
An assessment was completed by tissue viability Nurse (TVN) following extensive debridement of patients left leg. The leg wound started on the dorsum of the foot to below knee, fasciotomy medially exposing muscle, tendon, ligaments, fascia, total length 40cm circumferential (Figure 7, 8 & 9). The patient was ventilated and unstable. Pain was expressed during dressing changes from facial expressions and analgesia was increased before continuing the procedure. Plastics team opinion requested, TVN discussed options with plastics team.
The wound bed surface had slough, and exposed tendon, muscle, and no granular tissue. Referred to clinical nurse advisors from KCI for joint assessment for V.A.C. VERAFLO™ Therapy. The patient was being treated for ongoing sepsis with antibiotics and it was apparent the infection was still a risk to this patient. The patient had been to theatre twice and surgeons were keen to manage out of theatre. V.A.C. VERAFLO™ Therapy was the preferred option for the viability of this patient’s leg healing.
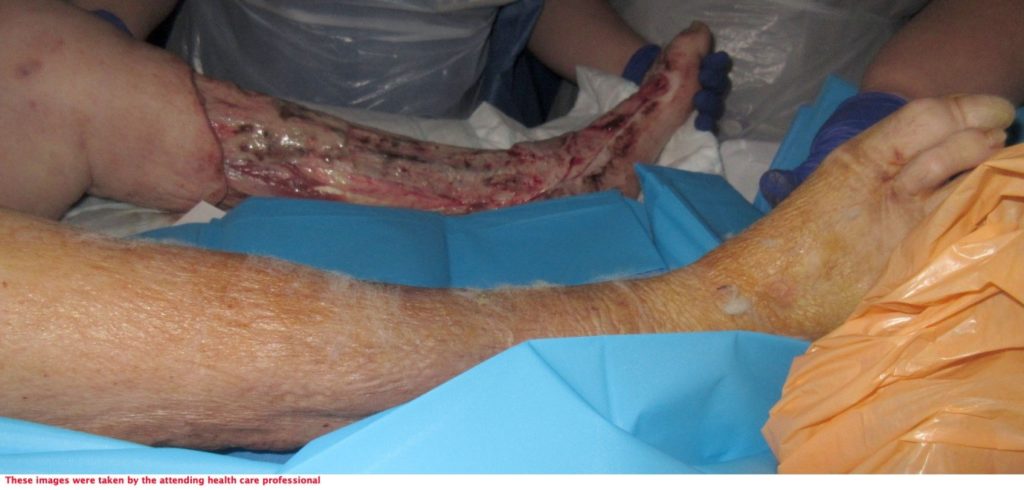
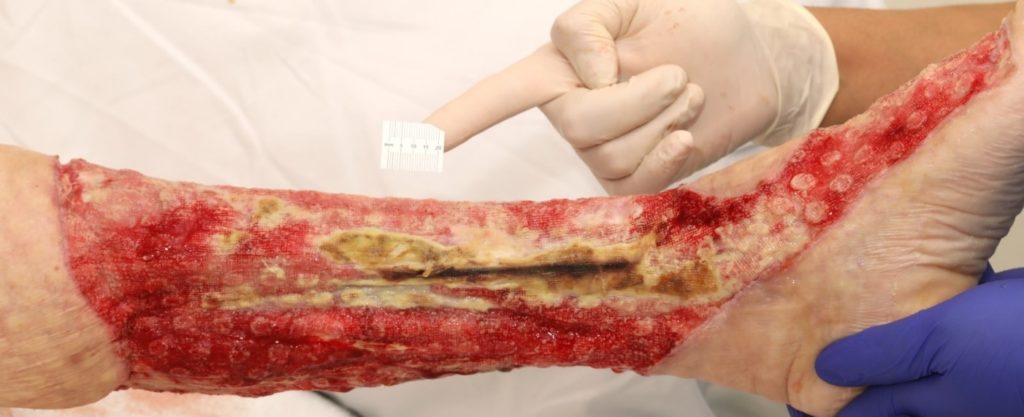
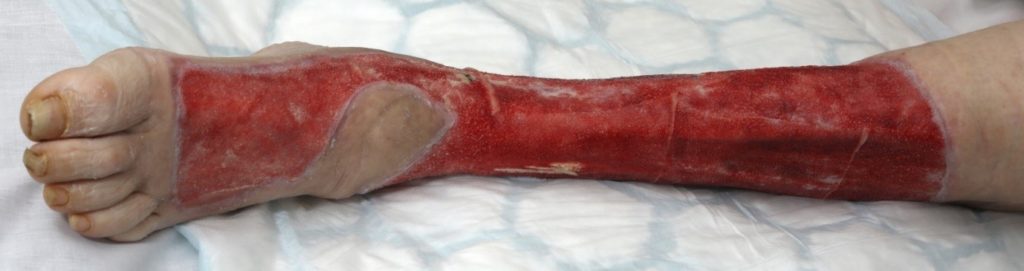
The severity of the non-viable tissue is captured in Figure 10 & 11. This is prior to V.A.C. VERAFLO™ application.
Commenced V.A.C. VERAFLO™ Therapy with challenges of patient pain and anxiety. Dressing changes initially took 2-3 hours by 2 TVNs and a clinical advisor from KCI or ward staff member. The therapy was used for a 10-week period.
V.A.C. VERAFLO CLEANSE CHOICE x 5 pieces were used, 3 x per week with V.A.C. VERAT.R.A.C. Duo™ Pad. Saline solution was instilled for a cycle of 10mins with 2-hour negative pressure. Hydrocolloid and 3M™ Cavilon™ No Sting Barrier Film (contained in V.A.C. VERAFLO™ dressing kit) were applied to Periwound and silicone contact layer to protect ligaments and muscle.
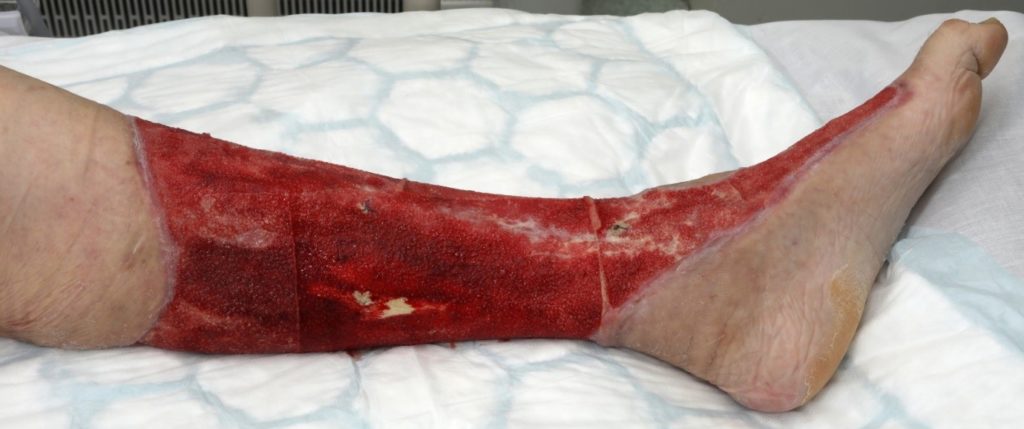
No further theatre debridement’s were required. After 9 days of treatment with V.A.C. VERAFLO CLEANSE CHOICE Significant granulation achieved. (figure 12 & 13)
TVN requested referral to pain specialist team and requested ward doctors to review for anxiety. Analgesia reviewed and Entonox used during dressing changes. Started on PRN analgesia and anti-anxiety medication prior to dressing changes. Patient’s favourite music was played at dressing changes to reduce anxiety and a nurse would be holding conversation with patient as a diversional therapy. Wound dressing changes continued, and progress with wound healing captured after 16days (figure 14,15 & 16)
After 4 Week and 4 day (32 days) of V.A.C. VERAFLO™ Therapy substantial levels of granulation tissue were observed (Figure 17, 18 & 19). TVN informed plastics consultant when granulation tissue appeared ready for a skin graft. Patient displayed low moods; requested depression score /assessment from ward doctors as it was so important to manage this pats anxiety as her pain was heightened by her anxiety and this could have comprised the use of V.A.C. VERAFLO™.
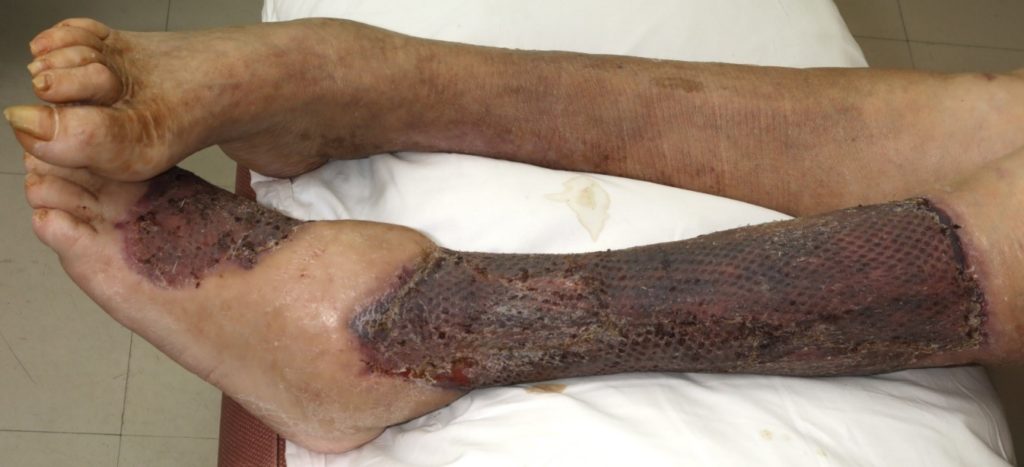
Skin graft performed and was successful (Figure 20, 21). After 14 days the patient was transferred to west park hospital for rehabilitation and subsequently went home with a care package in place.
Clinical Outcomes/ Conclusion:
V.A.C. VERAFLO™ Dressings have been innovatively designed to cleanse contaminated wounds, by helping to soften, solubilize, and remove viscous exudate, wet slough, fibrin, and other infectious materials by instilling a saline into the dressing, allowing it to soak and then gentle vacuum to remove the exudate.
It is my impression that due to V.A.C. VERAFLO™ Therapy the patient avoided a possible amputation. No further debridement was required in theatre post V.A.C. VERAFLO™ Therapy application, sepsis was controlled and improved treated during the duration of treatment.
TVNs recognised the importance of a holistic approach and supported patients’ anxieties well during dressing changes, which we feel contributed the management of her pain. A collaborative approach with Plastic team and good communication with ward staff to administer PRN pain and anti-anxiety medication prior to TVN visit ensured optimum patient comfort and increase TVN efficiency.