Case Study
ABSTRACT:
Pressure sores are lesions resulting from tissue ischemia secondary to compression over a bony prominence. This case demonstrates the presence of sacral pressure ulcer with secondary infection in a bedridden patient with multiple comorbidities and high anesthetic risk as well as its management, using the aid of negative pressure wound therapy with instillation and a dwell time. Our goal is to demonstrate a new adjunctive therapy to surgery to treat common pathology in a patient with high morbidity, proposing a shorter hospital stay, a decrease in the number of surgical procedures under general anesthesia and less wound manipulation needed.
INTRODUCTION
Pressure sores are among the major causes of a plastic surgeon call at the hospital environment. This condition usually affects patients with limited mobility and / or sensitivity. The sacral region, in particular, is usually affected in bedridden patients with chronic or acute pathologies, along with multiple associated comorbidities and the presence of clinical instability as a background to their treatment, making it difficult to indicate extensive surgical procedures such as aggressive debridement, osteotomies of bony prominences, intestinal or urological shunts, etc. The surgical treatment usually initiates with the debridement. When the presence of devitalized tissue is small, the use of topical debridement may be attempted, but in large amounts of necrosis surgical debridement is necessary. Bedside procedure is useful, but limited positioning and hemostatic control often limit the outcome of this approach. The anesthetic approach of patients with sacral ulcers is challenging, especially in dorsal ulcers, which often require prone position, which in patients with neurological or cardiovascular pathologies is a risky procedure3.
Most recently we have seen included in the arsenal of complex wound treatment the use of negative pressure wound therapy with instillation and a dwell time (NPWTi) – V.A.C. VeraFlo™ Therapy, †V.A.C.® Therapy (KCI, an Acelity company, San Antonio, TX). Such treatment is indicated in wounds affecting patients with multiple comorbidities, complex wounds, invasive infection, biofilm, patients ASA (American Society of Anesthesiology) classification greater than 2, especially diabetic and immunosuppressed ones.
In this report we demonstrate the application of this therapy to a 79-year-old patient who had been bedridden for 5 months after ischemic stroke, with a reported history of acute myocardial infarction (AMI) 2 months before, with grade IV(Pressure Sore Advisory Panel Consensus Development Conference3) sacral ulcer as an adjunct to classic antibiotics treatment, positioning care and nutrition, as well as its rapid clinical and local improvement and definitive closure requiring a single procedure under general anesthesia.
METHODS
Female patient, 79 years old, suffered a ischemic stroke in the putaminal region 6 months ago, with left hemiparesis and totally dependent on basic activities of daily living as sequelae ever since. During previous hospitalization due to stroke, was developed a sacral pressure sore, not surgically addressed at the time due to the high surgical risk, determined by the recent event of acute myocardial infarction, diagnosed in the third month of that admission.
After the 4th month she was discharged for ulcer care and rehabilitation as an outpatient.
After 5 months, she presented with fever, respiratory distress, abdominal distension, pressure sore secretions increasing and an overall status impairment. She was referred to our service, diagnosed with sepsis from both pulmonary and cutaneous focuses with positive blood culture for Klebsiella pneumoniae.
On clinical examination, she was unresponsive, tachycardic and feverish. On integument examination, the presence of extensive sacral ulcer affecting the full thickness of soft tissue, along with a large amount of moist and fetid necrotic tissue (Fig. 1).
Measures for clinical control, admission to the intensive care unit, empirical antibiotic therapy (after culture results spectrum was adjusted), culture collection, nutritional support, humidity control, pneumatic mattress, frequent decubitus change and plastic surgery evaluation for the possibility of sacral necrosis debridement were the initial treatment measures.
Taking into account the urgent need for debridement as well as the cardiologic-anesthetic contraindication for surgery, an alternative approach was chosen, the application of vacuum therapy, instillation associated with the use of a macro fenestrated foam intended to drain thick secretions, debris and other non-viable tissues that would not be aspirated by the regular fenestrations. This choice of treatment preserved the patient from general anesthesia, use of local vasoactive amines (anesthetics with vasoconstrictors), bleeding risks and avoid prone position (application in lateral position).
The system chosen was negative pressure wound therapy with instillation and a dwell time (NPWTi) With V.A.C. UltaTM (KCI, Acelity Company, San Antonio, TX). V.A.C. Foam Large two-part VeraFloTM with V.A.C. VeraT.R.A.C. DuoTM. The entire skin near the wound was covered for protection with hydrocolloid plaques, leaving only the wound in contact with the foam. Intestinal transit was diverted by a rectal tube and the perianal area to the intergluteal groove was sealed by hydrocolloid paste (Fig. 2.). One of the foam plates was fenestrated creating space for collecting thick secretions, debris and necrosis plates and the second one was leaved intact and placed over the first one (Fig. 3).
The solution chosen for instillation was 0.9% NaCl saline, the volume was 65 ml; this volume was determined with the aid of the V.A.C. UltaTM, based on the volume required to return the foam to the volume prior to the application of negative pressure. The cycle time was 10 minutes of immersion and 8 hours of 125mmhg negative pressure therapy, the dressing was maintained for 4 days. During this period, laboratory inflammatory markers decreased and the fever peaks ceased after 48h.
The first dressing change was performed at bedside. Upon removal, the foam had secretions and necrotic tissue adhered to the macro fenestrated channels (Fig. 4), the malodorous was gone and no purulent secretion visible. Remarkably, the previously firm and adhered necrosis was now softened with many spurs, allowing for easy manual removal with gauze (Fig. 1). The granulation tissue showed improvement in the areas where necrosis did not advance (Fig. 1).
Therapy was maintained with foam changing every 7 days, applying the same irrigation, cycle and negative pressure parameters with 0.9% saline. In total 1 initial application and 3 changes computing 25 days of NPWTi. Non sharp debridement was performed at each exchange, with bedside gauze avoiding invasive procedures without the need for anesthesia or risk of bleeding. After removal of the last foam, there was no necrotic tissue impeding reconstruction (Fig. 5) and the patient\’s clinical condition was already stabilized. During the hospitalization period, the cardiology team thoroughly reevaluated the patient\’s cardiac function and released the reconstructive procedure under anesthesia, the ejection fraction was above 60% and there were no objective signs of sequelae of recent myocardial ischemic event, as described in the transfer chart.
The surgical procedure was performed under general anesthesia, in prone position with a 45-minute surgical time and 60-minute anesthetic, without complications. The technique of choice was a unilateral fasciocutaneous V-Y flap, with bursa removal without sacral osteotomy (due to increased morbidity and required surgical time) and application of PREVENA ™ Incision Management System device (KCI, an Acelity company, San Antonio, TX) (Fig.6).
PREVENA ™ was maintained for 7 days and after removal no areas of dehiscence or suffering were observed (Fig. 7).
Patient was discharged after 45 days of hospitalization and weekly outpatient follow-up for 60 days without local complications.
DISCUSSION
The occurrence of pressure sores in this patient follows the epidemiological patterns described in the literature1,3,5: Elderly bedridden patient with acute decompensated pathology, cardiac disease, neurological injury and distant infection.
Standard treatment: control of associated infections, cardiovascular stabilization, nutritional support, humidity control, pneumatic mattress and frequent decubitus change was instituted. However, a great doubt remained in the multidisciplinary team, where the balance of the risk regarding surgical debridement hung: a severely ill patient with a high anesthetic – surgical risk, however with a septic condition, one of the focus being the ulcer.
Due to the imminent need for local treatment of necrosis, negative pressure therapy associated with instillation with V.A.C UltaTM therapy system was applied as the main treatment according to indication trends in the treatment of necrotic and infected wounds1,3,7,8,9,10,11.
The application of the cyclic solution immersion method and subatmospheric therapy was, in our view, one of the major factors that allowed the non-surgical treatment of necrosis without local infectious worsening. Only 1 surgical procedure under anesthesia was required during the entire hospitalization, which was performed in due course, with definitive character.
Another important detail of this approach was the manipulation absence of the wound within 4 days after the first application and after this a single weekly manipulation, which facilitated care by the nursing staff, reduced the risks of this. procedure in this patient. The therapy was effective in softening the necrotic tissue and by cyclic subatmospheric pressure the irrigation managed to collect part of the necrosis and thick secretion in the fenestrated channels, avoiding local putrefaction. The granulation tissue obtained after 25 days drew attention due to the unfavorable clinic and newly controlled septic process, allowing the definitive flap reconstruction to be achieved within this period, without any complications in the evolution and outpatient follow-up.
CONCLUSION
Negative pressure wound therapy with instillation and a dwell time in this case was effective, the length of stay was not prolonged by the treatment of pressure sore and tissue necrosis. In addition, the need for multiple surgical approaches to remove necrotic tissue was eliminated and wound bed preparation for surgical reconstruction quickly obtained enabling a flap for coverage without ischemic or infectious complications.
An important fact to be described was the absence of dressing changes under anesthesia in the critical phase of sepsis with the patient still without hemodynamic parameters control and without complete cardiac evaluation, generating greater tranquility and proper time for the multidisciplinary team to prepare the patient for surgery, downsizing substantially the anesthetic risk.
Thus, we present the treatment of a common pathology in a critically ill patient, conducted effectively and quickly with a decrease in the number of anesthetic surgical approaches and consequent lower risk exposure due to the aid of negative pressure therapy and intermittent instillation.
FIGURES.
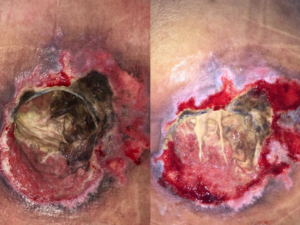
Fig.1: on the left: sacral region at the time of hospital admission. On the right: after 4 days of NPWTi.
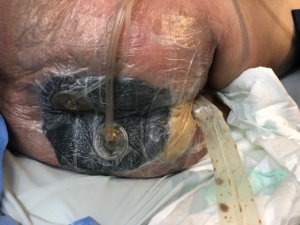
Fig. 2: Hydrocolloid ointment to aid hermetic closure of the dressing.
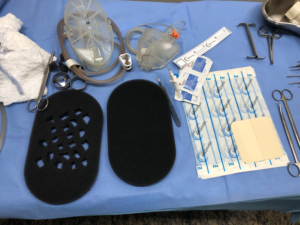
Fig. 3: Foam macro fenestration creating channels for debris and thick secretion drainage

Fig. 4: Foam appearance upon removal, necrotic eschar adhered to fenestrations.
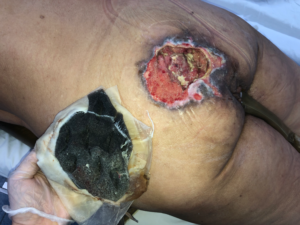
Fig. 5: Wound appearance after 25 days of NPWTi.
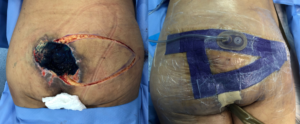
Fig. 6: Reconstruction procedure.
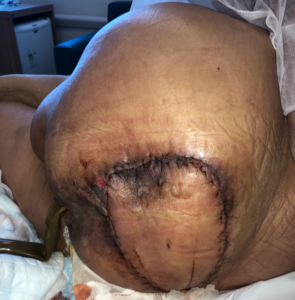
Fig. 7: Appearance after withdrawal PREVENA ™.
REFERENCES
- Cushing, Carolyn A.; Phillips, Linda G. Evidence-Based Medicine: Pressure Sores. Plast Reconstr Surg. 2013;132(6):1720-1732.
- Daniel RK, Wheatley D, Priest D. Pressure sores and paraplegia: An experimental model. Ann Plast Surg. 1985;15:41–49.
- Bauer J, Phillips LG. MOC-PSSM CME article: Pressure sores. Plast Reconstr Surg. 2008;121(Suppl):1–10
- Reddy M, Gill SS, Rochon PA. Preventing pressure ulcers: A systematic review. JAMA 2006;296:974–984.
- Rubayi S, Chandrasekhar BS. Trunk, abdomen, and pressure sore reconstruction. Plast Reconstr Surg 2011;128:201e-15e.
- Omar, M. Gathen, E. Liodakis, E.M. Suero, C. Krettek, C. Zeckey, and M Petri. A comparative study of negative pressure wound therapy with and without instillation of saline on wound healing. Journal of Wound Care 2016 25:8, 475-47
- Back DA, Scheuermann-Poley C, Willy C. Recommendations on negative pressure wound therapy with instillation and antimicrobial solutions – when, where and how to use: what does the evidence show?. Int Wound J. 2013 Dec;10 Suppl 1:32-42.
- Huang C, Leavitt T, Bayer LR, Orgill DP. Effect of negative pressure wound therapy on wound healing. Curr Probl Surg. 2014;51(7):301-331.
- Kim PJ, Attinger CE, Olawoye O, et al: Negative-pressure wound therapy with instillation: international consensus guidelines. Plast Reconstr Surg 2013;132:1569-1579.
- Fernandez L, Ellman C, Jackson P. Initial experience using a novel reticulated open cell foam dressing with through holes during negative pressure wound therapy with instillation for management of pressure ulcers. J Trauma Treat. 2017;6(5):410.
- Teot L, Boissiere F, Fluieraru S (2017) Novel foam dressing using negative pressure wound therapy with instillation to remove thick exudate. Int Wound J 14: 842-848.